316L Stainless Steel Water Tank for Pharmaceutical Grade Water



316L Stainless Steel Water Tank for Pharmaceutical Grade Water
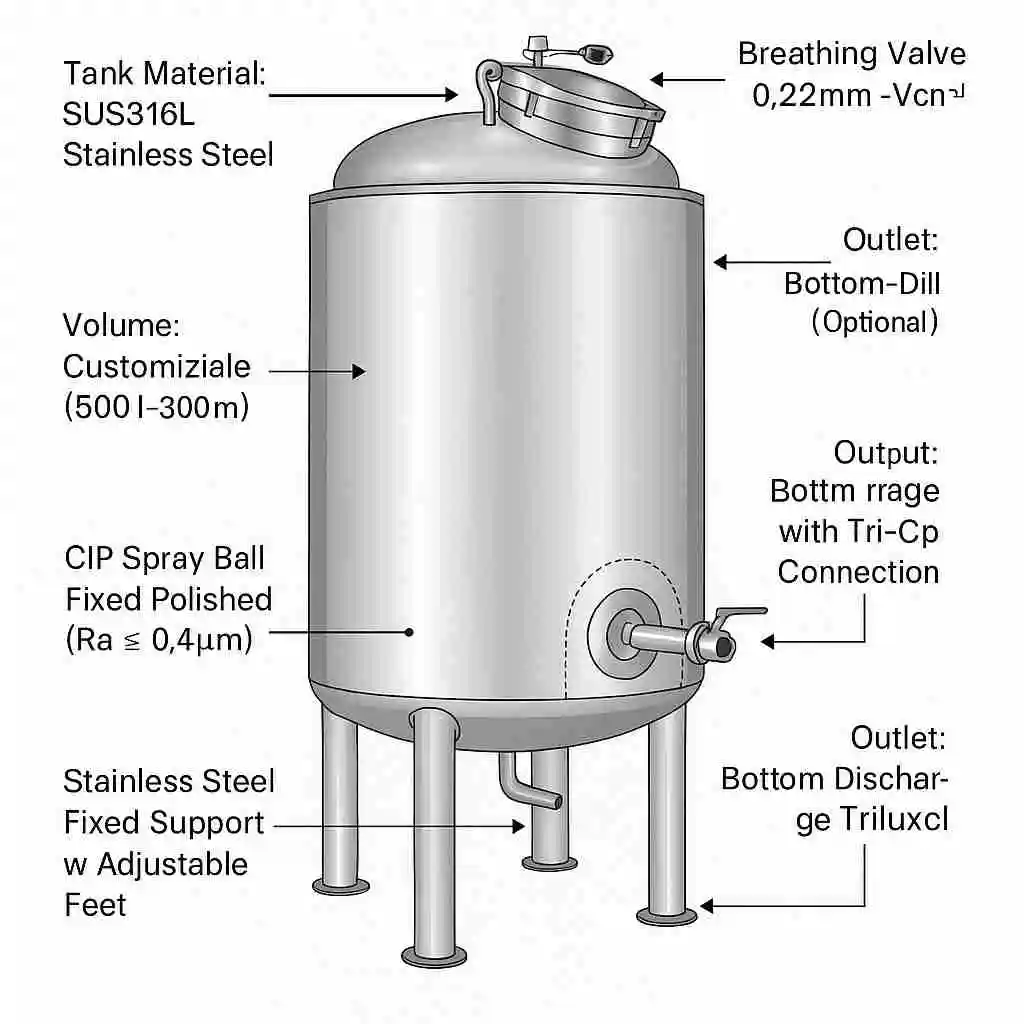
Designed for the most demanding purity requirements, this pharmaceutical-grade water tank is constructed from high-quality SUS316L stainless steel. Engineered for GMP-compliant environments, it features a fully customizable design, including CIP cleaning ports, sanitary breathing valves, and a mirror-polished interior surface for optimal hygiene and corrosion resistance.
Designed for the most demanding purity requirements, this pharmaceutical-grade water tank is constructed from high-quality SUS316L stainless steel. Engineered for GMP-compliant environments, it features a fully customizable design, including CIP cleaning ports, sanitary breathing valves, and a mirror-polished interior surface for optimal hygiene and corrosion resistance.
The STARK 316L stainless steel water tank for pharmaceutical applications is meticulously designed for ultra-pure water storage and distribution in critical environments such as cleanrooms, sterile process lines, and injectable solution systems. Built with superior SUS316L stainless steel, the tank ensures excellent corrosion resistance against high-purity water and aggressive cleaning agents.
All interior contact surfaces are mirror-polished to Ra ≤ 0.4 μm, greatly minimizing bacterial adhesion and ensuring optimal cleanability. The tank construction strictly follows GMP design principles, including dead-leg-free geometry, crevice-free welds, and full drainability to eliminate residue accumulation.
Sanitary-Grade Components
To support the stringent operational needs of pharmaceutical-grade water systems (PW, WFI), each tank is equipped with the following high-spec components:
- CIP Spray Ball: Fixed or rotary-type clean-in-place spray device for full 360° interior washing coverage, compatible with automated CIP systems.
- Sanitary Breathing Valve: Ensures filtered air exchange while protecting the interior from microbial ingress.
- Sight Glass with LED Illumination: Visual inspection window with optional light port for real-time level and clarity monitoring.
- Vent Filter Housing Port: Compatible with 0.22μm sterilizing grade filters for pharmaceutical ventilation control.
- Level Sensor: Capacitance or ultrasonic level detection interface with optional 4–20 mA output.
- Manway: Top or side-mounted sanitary manhole with safety interlock clamp for easy access and maintenance.
- Outlet Valve Options: Available with diaphragm valve, butterfly valve, or bottom drain valve—all sanitary-grade and removable without tools.
Custom Engineering
STARK provides full customization for each pharmaceutical-grade tank, including but not limited to:
- Tank volume: from 500L to 30,000L or fully customized
- Design pressure: atmospheric or pressurized (up to 0.6 MPa)
- Jacketed construction for thermal insulation or temperature control
- Multi-layer insulation options: polyurethane, rock wool, or vacuum panels
- Electropolished inner surface for ultrapure or WFI-grade water
- Leg type: fixed stand, skirt base, or mobile trolley
Compliance & Quality Assurance
All fabrication and welding processes are executed by certified professionals under a strict ISO 9001 quality system. Each tank is hydro-tested and undergoes dimensional, surface finish, and weld seam inspection. Upon request, STARK can provide complete documentation packages including:
- Material Test Certificates (EN 10204 3.1)
- Welding Qualification Records (WPS/PQR)
- Surface Roughness Validation Report
- Pressure Testing and Inspection Report
- FAT Protocols and Photos
This tank is ideal for use in purified water (PW), water for injection (WFI), clean utility loops, and as a buffer tank for sterile process systems. With exceptional build quality and pharmaceutical-grade configuration, it helps facilities meet stringent FDA, EU GMP, and WHO water standards.
| Parameter | Specification |
|---|---|
| Material | SUS316L Stainless Steel (contact surface) |
| Surface Finish (Inner) | Mirror Polished, Ra ≤ 0.4 μm (electropolishing optional) |
| Surface Finish (Outer) | Brushed, Polished, or Matte (customizable) |
| Design Volume | 500L – 30,000L (custom sizes available) |
| Design Pressure | Atmospheric / Pressurized (up to 0.6 MPa) |
| Working Temperature | -10°C to +120°C (with insulation option) |
| Structure Type | Vertical Cylindrical (horizontal or mobile optional) |
| Manhole | Top or side-mounted, sanitary-grade |
| Inlet / Outlet Connection | Clamp, flange, or threaded (customizable) |
| Standard Accessories | CIP spray ball, breathing valve, sight glass, level sensor, thermometer |
| Optional Features | Insulation layer, jacketed heating/cooling, caster wheels, load cells |
| Compliance | GMP, FDA, USP, EP, ISO 9001 |
Note: All technical specifications can be tailored to customer-specific requirements. For detailed drawings, P&ID integration, or pressure vessel code compliance (ASME/CE), please contact us.
This pharmaceutical-grade 316L stainless steel tank is purpose-built for use in validated clean environments, where microbiological purity, chemical compatibility, and process stability are mission-critical. It plays a vital role in the following applications:
- Purified Water (PW) Storage: Maintains stable, low-conductivity water for non-injectable manufacturing processes, meeting USP/EP standards.
- Water for Injection (WFI) Buffer Tank: Used as a sterile storage and buffer unit in WFI loops, featuring electropolished surfaces and sanitary design to prevent endotoxin formation.
- Clean Utility Distribution: Supports cleanroom-grade water circulation systems with automated monitoring and pressure regulation features.
- Vaccine or IV Solution Preparation: Serves as a buffer tank in upstream or downstream stages of biopharmaceutical formulation, including sterile mixing and bulk holding.
- Intermediate Ingredient Storage: Ideal for holding filtered raw liquids before aseptic filling or further downstream processing.
- CIP Return or Drain Collection: Collects clean-in-place rinse or recirculated sanitizing fluids within GMP-grade process lines.
Widely deployed in pharmaceutical plants, biotech R&D facilities, medical device cleanrooms, and high-purity water system integrators, this tank solution helps ensure batch safety, regulatory compliance, and uninterrupted clean utility supply.
The STARK 316L stainless steel water tank for pharmaceutical applications is meticulously designed for ultra-pure water storage and distribution in critical environments such as cleanrooms, sterile process lines, and injectable solution systems. Built with superior SUS316L stainless steel, the tank ensures excellent corrosion resistance against high-purity water and aggressive cleaning agents.
All interior contact surfaces are mirror-polished to Ra ≤ 0.4 μm, greatly minimizing bacterial adhesion and ensuring optimal cleanability. The tank construction strictly follows GMP design principles, including dead-leg-free geometry, crevice-free welds, and full drainability to eliminate residue accumulation.
Sanitary-Grade Components
To support the stringent operational needs of pharmaceutical-grade water systems (PW, WFI), each tank is equipped with the following high-spec components:
- CIP Spray Ball: Fixed or rotary-type clean-in-place spray device for full 360° interior washing coverage, compatible with automated CIP systems.
- Sanitary Breathing Valve: Ensures filtered air exchange while protecting the interior from microbial ingress.
- Sight Glass with LED Illumination: Visual inspection window with optional light port for real-time level and clarity monitoring.
- Vent Filter Housing Port: Compatible with 0.22μm sterilizing grade filters for pharmaceutical ventilation control.
- Level Sensor: Capacitance or ultrasonic level detection interface with optional 4–20 mA output.
- Manway: Top or side-mounted sanitary manhole with safety interlock clamp for easy access and maintenance.
- Outlet Valve Options: Available with diaphragm valve, butterfly valve, or bottom drain valve—all sanitary-grade and removable without tools.
Custom Engineering
STARK provides full customization for each pharmaceutical-grade tank, including but not limited to:
- Tank volume: from 500L to 30,000L or fully customized
- Design pressure: atmospheric or pressurized (up to 0.6 MPa)
- Jacketed construction for thermal insulation or temperature control
- Multi-layer insulation options: polyurethane, rock wool, or vacuum panels
- Electropolished inner surface for ultrapure or WFI-grade water
- Leg type: fixed stand, skirt base, or mobile trolley
Compliance & Quality Assurance
All fabrication and welding processes are executed by certified professionals under a strict ISO 9001 quality system. Each tank is hydro-tested and undergoes dimensional, surface finish, and weld seam inspection. Upon request, STARK can provide complete documentation packages including:
- Material Test Certificates (EN 10204 3.1)
- Welding Qualification Records (WPS/PQR)
- Surface Roughness Validation Report
- Pressure Testing and Inspection Report
- FAT Protocols and Photos
This tank is ideal for use in purified water (PW), water for injection (WFI), clean utility loops, and as a buffer tank for sterile process systems. With exceptional build quality and pharmaceutical-grade configuration, it helps facilities meet stringent FDA, EU GMP, and WHO water standards.
| Parameter | Specification |
|---|---|
| Material | SUS316L Stainless Steel (contact surface) |
| Surface Finish (Inner) | Mirror Polished, Ra ≤ 0.4 μm (electropolishing optional) |
| Surface Finish (Outer) | Brushed, Polished, or Matte (customizable) |
| Design Volume | 500L – 30,000L (custom sizes available) |
| Design Pressure | Atmospheric / Pressurized (up to 0.6 MPa) |
| Working Temperature | -10°C to +120°C (with insulation option) |
| Structure Type | Vertical Cylindrical (horizontal or mobile optional) |
| Manhole | Top or side-mounted, sanitary-grade |
| Inlet / Outlet Connection | Clamp, flange, or threaded (customizable) |
| Standard Accessories | CIP spray ball, breathing valve, sight glass, level sensor, thermometer |
| Optional Features | Insulation layer, jacketed heating/cooling, caster wheels, load cells |
| Compliance | GMP, FDA, USP, EP, ISO 9001 |
Note: All technical specifications can be tailored to customer-specific requirements. For detailed drawings, P&ID integration, or pressure vessel code compliance (ASME/CE), please contact us.
This pharmaceutical-grade 316L stainless steel tank is purpose-built for use in validated clean environments, where microbiological purity, chemical compatibility, and process stability are mission-critical. It plays a vital role in the following applications:
- Purified Water (PW) Storage: Maintains stable, low-conductivity water for non-injectable manufacturing processes, meeting USP/EP standards.
- Water for Injection (WFI) Buffer Tank: Used as a sterile storage and buffer unit in WFI loops, featuring electropolished surfaces and sanitary design to prevent endotoxin formation.
- Clean Utility Distribution: Supports cleanroom-grade water circulation systems with automated monitoring and pressure regulation features.
- Vaccine or IV Solution Preparation: Serves as a buffer tank in upstream or downstream stages of biopharmaceutical formulation, including sterile mixing and bulk holding.
- Intermediate Ingredient Storage: Ideal for holding filtered raw liquids before aseptic filling or further downstream processing.
- CIP Return or Drain Collection: Collects clean-in-place rinse or recirculated sanitizing fluids within GMP-grade process lines.
Widely deployed in pharmaceutical plants, biotech R&D facilities, medical device cleanrooms, and high-purity water system integrators, this tank solution helps ensure batch safety, regulatory compliance, and uninterrupted clean utility supply.














